The Relationship Between Chromite Ore and Ferrochrome Production
Introduction:Chromite Ore and Ferrochrome
In simple terms, ferrochrome is an alloy of chromium and iron containing between 50-70% chromium. Creating ferrochrome from chromite ore is quite an art. This transformation is achieved through a smelting process in electric arc furnaces. The resulting ferrochrome is a game-changer. Once it’s blended into steel, it boosts the steel’s resistance to corrosion, oxidation, and even gives it a shiny appearance. In this article, we explore the relationship between chromite ore and ferrochrome:
Buy chromite ore in different grades from Iran Chromite Group, offered in lumps or fines. We will supply various inspection reports, including SGS and CIQ reports, in addition to a reliable contract. Our access to top mines and facilities in Iran enables us to provide limitless supplies of chromite ore in lumpy or fine form.
What Is the Difference Between Ferro Chrome and Chrome Ore?
First off, chrome ore is essentially a combination of iron and chromium oxides, primarily consisting of the mineral chromite. This natural ore is mined directly from the earth. Read What is Chromite Ore and Where is it Found?
Historically, chromite ore was discovered in the early 19th century. People back then realized that when this ore was heated, it produced a metallic element, chromium. This element was shiny, corrosion-resistant and found its use in several applications – from protective coatings to dyes.
Now, when we talk about ferrochrome, we’re referring to an alloy – which is a fancy term for a mixture of metals. Ferrochrome is a blend of iron and chromium, with a sprinkle of carbon and other elements. By heating chromite ore in the presence of carbon, ferrochrome is produced. This alloy is incredibly useful as it lends its strength and anti-corrosive properties to stainless steel.
Here’s where it gets really interesting. Initially, the production methods for ferrochrome were quite basic. But over the years, man, the changes have been remarkable! There’s been a tremendous evolution in technology and processes. From basic open-hearth methods, we’ve moved to sophisticated electric furnaces, enhancing the efficiency and quality of ferrochrome production.
Ferrochrome: An Essential Alloy
Ferrochrome, in the simplest terms, is an alloy that consists primarily of chromium and iron. Imagine it as a symbiotic relationship where both these elements come together to create something more potent and versatile. The iron provides strength, and the chromium imparts corrosion resistance – a perfect marriage, if you ask me!
Now, don’t get it twisted – not all Ferrochrome is created equal. Over the years, I’ve come to learn about its various versions. There’s High Carbon Ferrochrome (HCFeCr), Medium Carbon Ferrochrome (MCFeCr), and Low Carbon Ferrochrome (LCFeCr). The difference? Well, it’s mainly the carbon content. HCFeCr, for instance, has a high carbon percentage, usually over 4%. LCFeCr, on the other hand, contains less than 0.10% carbon. Depending on the application, one might choose one variant over the other. It’s all about striking the right balance!
The role of Ferrochrome in stainless steel production
You see, stainless steel, that shiny, rust-resistant metal we love so much – can’t be produced without Ferrochrome. When chromium is added to steel, magic happens. The chromium forms a protective layer of chromium oxide on the steel surface. This layer is so thin, it’s almost invisible! It’s super effective at preventing iron from oxidizing (or rusting). That’s the very reason our kitchen sinks, cutlery, and even some of our favorite architectural landmarks, like the Cloud Gate in Chicago, remain so brilliantly shiny and rust-free!
For the best results, you want the chromium from Ferrochrome. The alloy ensures that the chromium disperses evenly throughout the steel, giving it that uniform, shiny appearance and incredible corrosion resistance.

The Production Process of Chrome Ore and FerroChrome:
Extracting Chromite Ore from the earth
Let me paint a picture for you: vast open-pit mines, enormous earth-moving machines, and the distinct scent of freshly dug earth. That’s where the story of chromite begins. First, we identify the areas where chromite is concentrated, usually through geological surveys.
Once we’ve pinpointed the location, it’s all about carefully planning the extraction. We need to decide if we’re going for an open-pit method or underground mining. Most often, if the ore is close to the surface, open-pit is the way to go. We strip away the overburden – that’s the fancy term for the stuff above the chromite layer – and then get digging.
Massive machines, scooping up tons of earth in one go, and revealing the shiny, dark chromite beneath. That’s how you get chromite ore out from the embrace of the earth. Read More:Methods of Chromite Ore Extraction
From Chromite Ore to Ferrochrome: A step-by-step guide
Step 1: Crushing and Grinding. First, the ore needs to be crushed down to a manageable size. This helps in increasing the surface area and is essential for the next steps. After crushing, it’s grinding time. Imagine a super-sized blender mixing and turning those chunks into a fine powder.
Step 2: Beneficiation. Beneficiation is essentially a purification process. We use various techniques – like gravity concentration or magnetic separation – to increase the chromite content.
Step 3: Smelting. Here, things get really hot – literally. We take the ore, mix it with coke (It’s a type of coal) and a few other ingredients and then heat it all up in an electric furnace. The temperatures? A sizzling 2,800°F (1,540°C) or more!
Step 4: Formation of Ferrochrome. As the mixture melts, the chromite reacts with the coke, and voilà – ferrochrome is born. This silvery, metallic alloy is what gets used in all kinds of stuff, from stainless steel to those shiny car parts.
Economic Implications of Chromite Ore
The global market value of Chromite and Ferrochrome
Over the past few years, the global market value of chromite and its derivative, ferrochrome, has been on a steady rise.
The steel industry, in particular, owes a massive chunk of its operations to these two. When you alloy chromite with iron, you get ferrochrome – and this bad boy is essential in stainless steel production.
The beauty of stainless steel! It’s everywhere – from kitchen sinks to skyscrapers – and guess what? None of it would be possible without our humble chromite ore. The global demand for stainless steel has caused the value of chromite and ferrochrome to skyrocket. And as urbanization and infrastructure development progress, especially in emerging economies, this demand is only going to increase.
Major players in the Chromite and Ferrochrome industry
South Africa is hands down the biggest player, with the lion’s share of the world’s chromite reserves. Every time I visited there, I was awestruck by the sheer scale of their operations. Kazakhstan and India aren’t too far behind, making significant contributions to the global output.
But it’s not just about the countries; several global corporations are deeply entrenched in this trade. Their influence is palpable, from setting chromite prices to establishing global supply chains. If you ever find yourself at an international minerals conference, you’ll likely hear murmurs and whispers about these giants, discussing their latest ventures and projects.
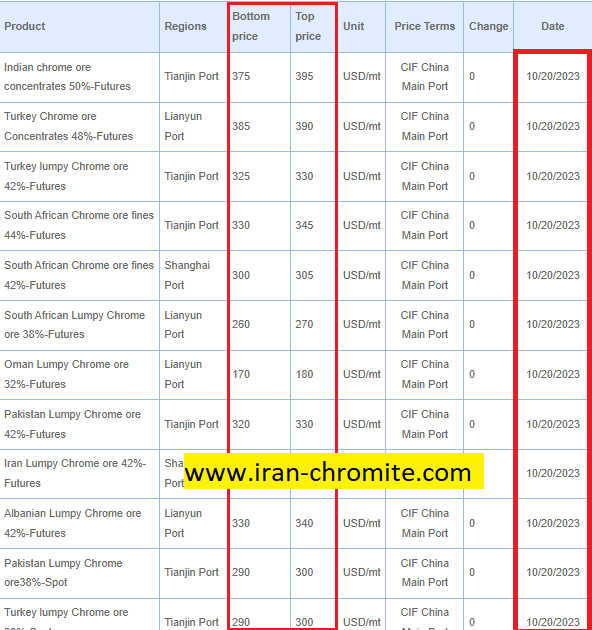
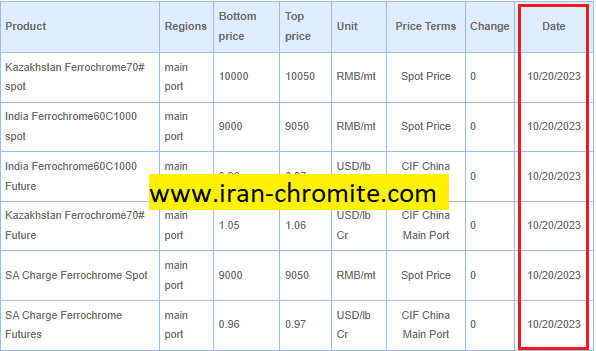
Conclusion:Chromite Ore and Ferrochrome
Ferrochrome, an alloy comprising 50-70% chromium and iron, is derived from chromite ore through a smelting process in electric arc furnaces. This alloy enhances steel’s resistance to corrosion and oxidation and imparts a shiny finish.
Variants of Ferrochrome include High, Medium, and Low Carbon Ferrochrome, which differ in carbon content. Essential for stainless steel production, Ferrochrome allows chromium to evenly disperse, ensuring rust-resistance.
With rising global demand for stainless steel, the value of chromite and ferrochrome has surged, with South Africa being the leading producer.
